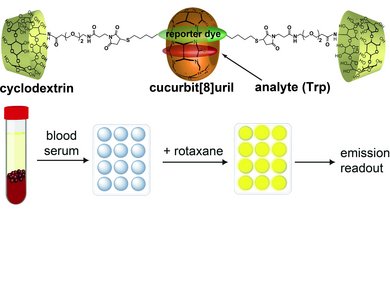
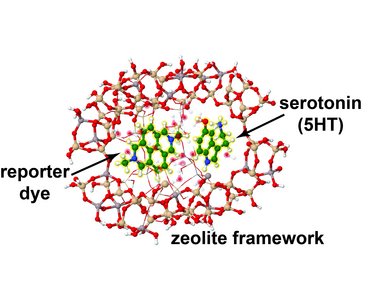
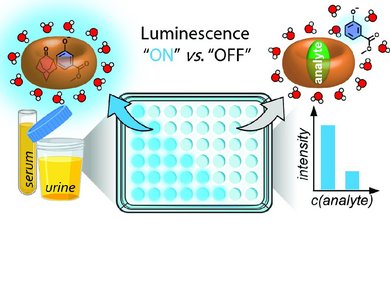
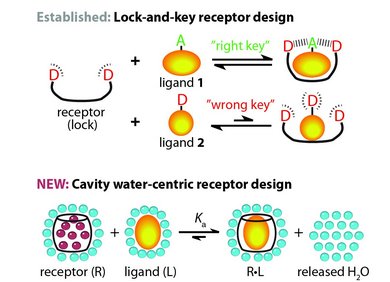
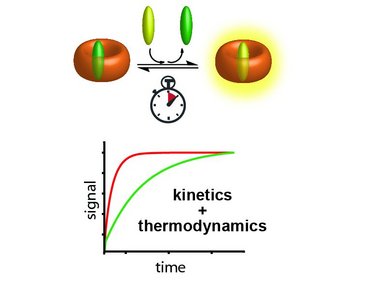
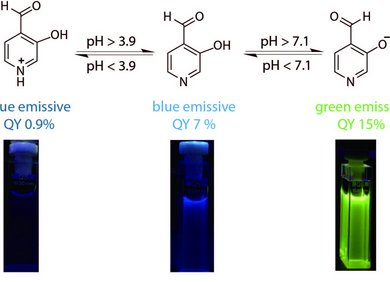
This line of research focuses on the development of dual macrocyclic rotaxanes, offering unique sensing opportunities in complex biofluids like blood serum where traditional supramolecular systems have faced challenges in sensitivity and selectivity. Utilizing the macrocycle cucurbit[8]uril, we capitalize on its capacity to incorporate two molecules within its cavity, promoting close interaction between a reporter dye and a target analyte (e.g., aromatic amino acids like Trp and Phe). This molecular proximity facilitates electronic coupling, yielding sensitive readouts such as emission changes. Furthermore, the bulky stopper groups prevent the disassembly of CB8-dye rotaxanes in saline media, such as biofluids, which is essential for their use in real sensing applications.
Nat. Commun. 2023, 14, 518; link.